This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
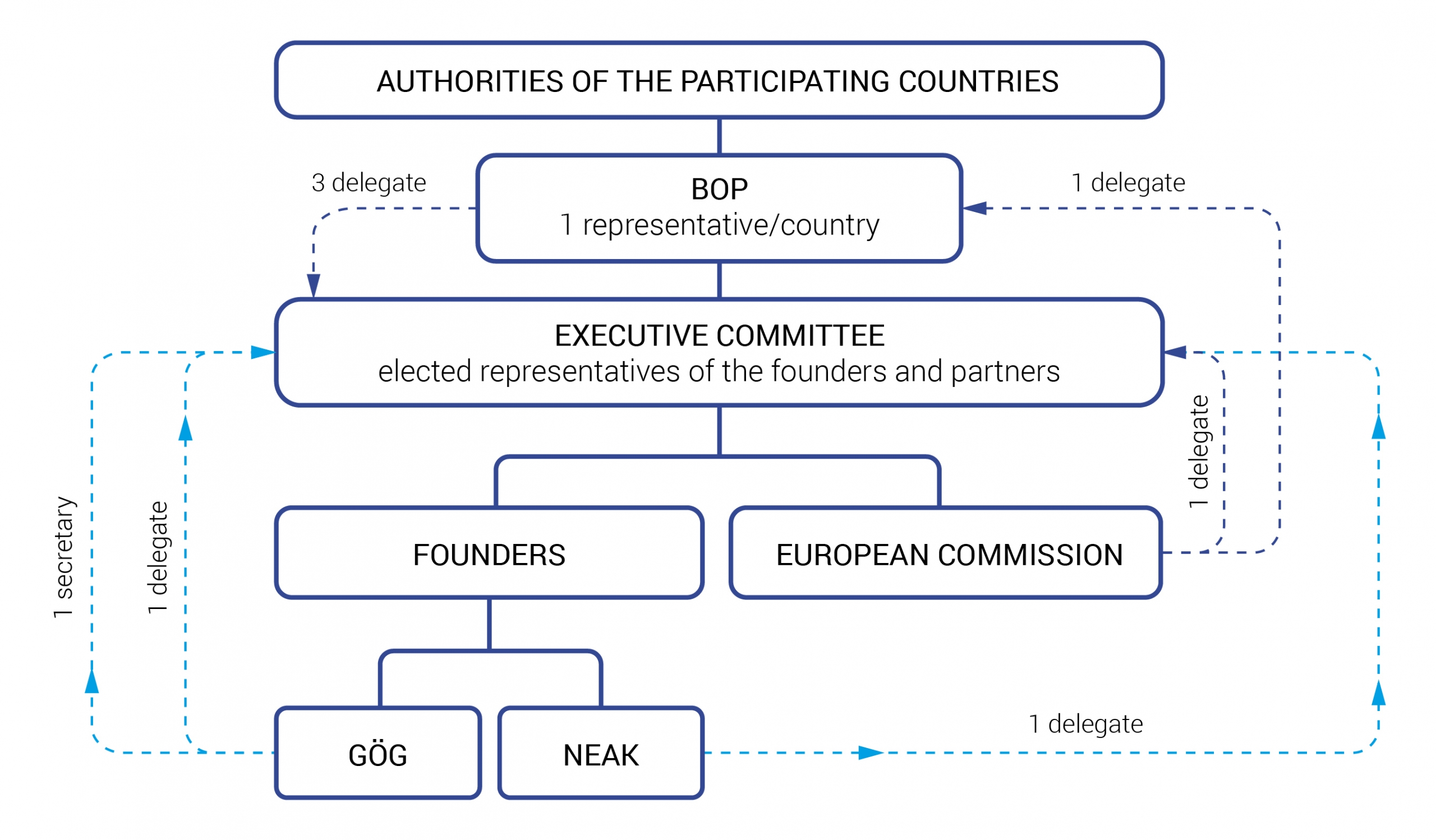
The EURIPID collaboration has three main bodies: (1) the Board of Participants (BoP), (2) the Executive Committee and the project team. The composition and responsibilties of the three bodies are as follows:
The EURIPID collaboration has three main bodies: the Board of Participants (BoP), the Executive Committee and the project team.
Board of Participants (BoP)
The BoP consists of one representative of each participating country or organisation and the members of the Executive Committee, which are not part of the BoP. The BoP carries out strategic decisions. Each representative has a voting right and decisions are taken by simple majority. The chair of the BoP is elected by the participants for three years. The BoP has at least one annual meeting.
Executive Committee
One representative of each founder (NEAK and GÖG), three elected members of the EURIPID collaboration and a representative of the European Commission are members of the Executive Committee. Members of the Executive Committee are also members of the BoP. The responsibilities of the Executive Committee focus on the operational level: the operationalisation of the BoP’s strategic decision, to take high-level operational decisions, to carry out operational work and to monitor developments in this area closely in order to report them at the annual BoP meeting.
Project team
The project team acts on behalf of the members and is responsible for project management and all activities related to smooth operating of the project. Experts of the founders, NEAK and GÖG, are responsible for carrying out the daily work (e.g. webpage and database maintenance).
The legal framework consists of the Framework Partnership Agreement, the General Terms and Conditions to the Framework Partnership Agreement of EURIPID Collaboration (GTC) and the Terms and Conditions of Use of the EURIPID Website (TCU).
Chair of the Board of Participants
Momir Radulović
Agency for Medicinal Products and Medical Devices of the Republic of Slovenia (JAZMP)
Executive Director of the Slovenian Regulatory Authority. His previous work experience includes hospital and community pharmacy and the pharma industry.
Executive Committee
Gergely Németh
Project Manager
National Institute of Health Insurance Fund Management
(Nemzeti Egészségbiztosítási Alapkezelő, NEAK)
Head of EURIPID project
Claudia Habl
General Secretary of the Board of Participants
Austrian National Public Health Institute
(Gesundheit Österreich Gmbh, GÖG)
Head of Dept. International Affairs and Consulting, working on pharmaceutical pricing for more than 20 years
Francis Arickx
Chair of the Executive Committee
National Institute for Health and Disability Insurance (Rijksinstituut voor ziekte- en invaliditeitsverzekering, RIZIV INAMI)
Head of the directorate Pharmaceutical Policy.
Marie Orre
Member of the Executive Committee
The Dental and Pharmaceutical Benefits Agency (Tandvårds- och läkemedelsförmånsverket, TLV)
Analyst at the TLV. Background as a medical researcher and analyst within the pharmaceutical industry.
Sónia Caldeira
Member of the Executive Committee
Infarmed (Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.)
Senior economist at Health Technology Assessment and Pricing Department
Member of the Executive Committee
Representative from the European Commission
Directorate-General for Health and Food Safety
Unit B4 for Medical products: quality, safety, innovation
Email : SANTE-ATF@ec.europa.eu