This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Access to EURIPID data for researchers
The EURIPID Collaboration is ready to support the research of the academic sector in the field of medicinal product pricing and access to medicines. The below described procedure has to be followed.
Researchers are invited to register to the Newsletter.
Provision of EURIPID data to researchers
At first, an application form needs to be filled out by the applicant.
Confidential disclosure agreement: With the submission of the application form, the applicant agrees signs a confidential disclosure agreement and the EURIPID collaboration countersigns after a positive decision of the Board of Participants (BoP).
Agreement to share results with the Euripid-collaboration: With the submission of the application form, the applicant agrees to share the results of the research with the EURIPID collaboration.
After the applicant submitted the application, the EURIPID team reviews the application for completeness. Further, applications may require additional programming of SQL-specific queries which will be assessed by the EURIPID team. The EURIPID Executive Committee assesses complete applications with regard to content. If the Executive Committee endorses the application, a formal voting procedure by the BoP will be initiated.
The BoP is responsible for making a decision on applications on a case-by-case basis. The prodecure takes three weeks The applicant will be informed by the EURIPID team if the decision of the BoP was positive or negative. In case of a negative decision, the applicant will be informed by the EURIPID team on the reasons of rejecting the application. In case of a positive decision, the applicant will be informed by the EURIPID team on the further procedure.
4a – Notification & data extract: If the request can be fulfilled with available means, the EURIPID team informs the applicant about the positive decision of the BoP and provides the requested data extract.
4b – Query against payment: If the request cannot be fulfilled with available means and additional SQL programming is necessary, the EURIPID team informs the applicant to contact the information provision service of the Hungarian National Institute of Health Insurance Fund (NEAK). The information provision services initiates the necessary extra query against payment and provides the data extract.
The research team or individual researcher must sign a confidential disclosure agreement when applying for data access confirming that no product level data will be shared with Third parties. Additionally, the applicant has to sign an agreement that the results of the research will be shared with the EURPID collaboration. After a positive decision of the BoP, the EURIPID collaboration countersigns the Confidentiality Disclosure Agreement.
After the signature of the Confidentiality Disclosure Agreement and the agreement to share the results if the research, the applicant can start the research. The research should be strictly non-commercial and serve public interest, as defined in the WHA resolution on price transparency. Requests for bachelor or master-theses only shall be excluded.
If the applicant requested a quality check of the research based on EURIPID data in the application, the EURIPID team will conduct the quality review.
The researcher has to share the final analysis with the EURIPID collaboration for publication on the EURIPID public website once the article is published. The Euripid team reserves the right to be listed as co-author in publications, especially when a quality review of the analysis and/or input to the methodology was provided.
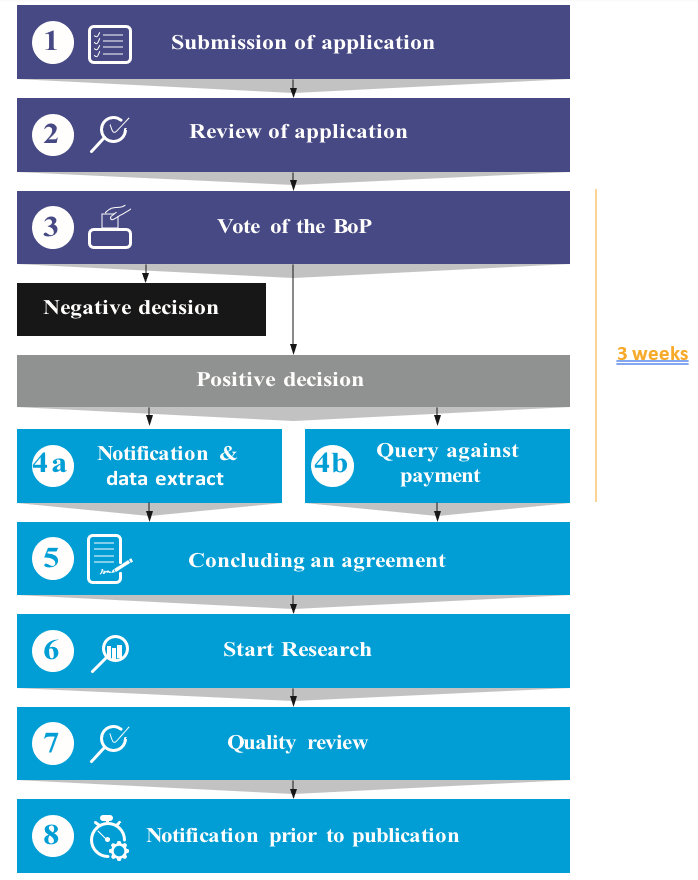
1) Submission of application form
At first, the application form which can be found at the bottom of the page has to be filled in and submitted by the applicant.
2) Review and assessment of application
After the applicant submitted the application the EURIPID team reviews the application for clarity and feasibility. If necessary the team goes back to the applicant. In the next step the EURIPID Executive Committee assesses the applications if it is from the academic sector and is of public interest. Requests for bachelor or master-theses shall be excluded. If the Executive Committee endorses the application, a formal voting procedure by the BoP will be initiated.
3) Vote on application by BoP
The BoP is responsible for making a decision on applications on a case-by-case basis. The prodecure takes three weeks The applicant will be informed by the EURIPID team if the decision of the BoP was positive or negative. In case of a negative decision, the applicant will be informed by the EURIPID team on the reasons of rejecting the application. In case of a positive decision, the applicant will be informed by the EURIPID team on the further procedure.
4) Data provision
After a positive decision of the BoP, there are two different procedures.
4a – Notification & data extract: If the request can be fulfilled with available means, the EURIPID team informs the applicant about the positive decision of the BoP and provides the requested data extract.
4b – Query against payment: If the request cannot be fulfilled with available means and additional SQL programming is necessary, the EURIPID team informs the applicant to contact the information provision service of the Hungarian National Institute of Health Insurance Fund (NEAK). The information provision services initiates the necessary extra query against payment and provides the data extract.
5) Concluding an agreement
The research institute must sign an agreement in which the service, the fee, the deadlines, confidentiality clauses and further obligations are settled. The results of the research shall be shared with and published by the EURPID Collaboration.
Please note that in case of information request from the European Union for the electronic signature of the agreement only electronic signatures from trust service providers delivering Qualified certificate for electronic signature are accepted which are listed on the following page:
https://esignature.ec.europa.eu/efda/tl-browser/#/screen/home.
In case of information request outside of the European Union options for electronic signature shall be approved on a case by case basis.
If electronic signature cannot be used for the signature of the agreement then it can be signed in paper format.
6) Start Research
After the signature of the Confidentiality Disclosure Agreement and the agreement to share the results if the research, the applicant can start the research. The research should be strictly non-commercial and serve public interest, as defined in the WHA resolution on price transparency. Requests for bachelor or master-theses only shall be excluded.
7) Quality Review (optional)
If the applicant requested a quality check of the research based on EURIPID data in the application, the EURIPID team will conduct the quality review.
8) Notification prior to publication
The researcher has to share the final analysis with the EURIPID collaboration for publication on the EURIPID public website once the article is published. The Euripid team reserves the right to be listed as co-author in publications, especially when a quality review of the analysis and/or input to the methodology was provided.
Researcher Application
Agreement between EURIPID and researchers
Here you can find the agreement on data provision.
